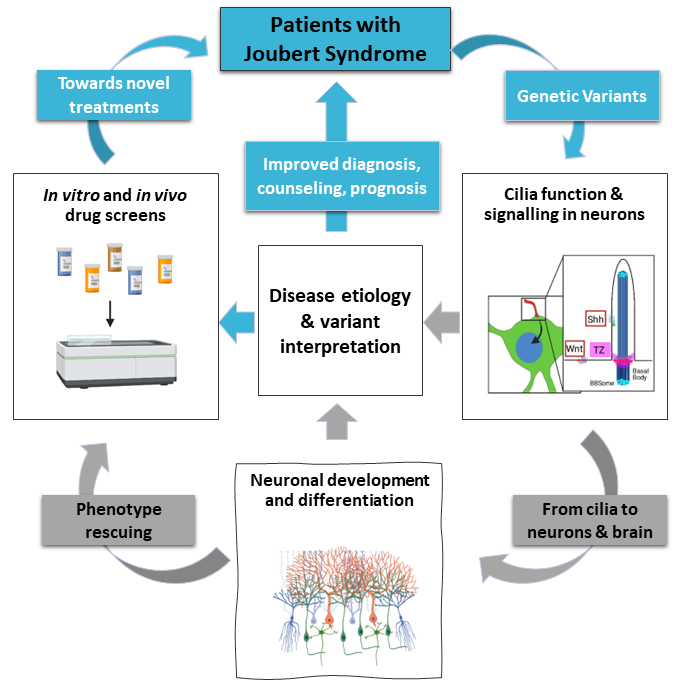
NDCil is an interdisciplinary research consortium of five partners across five countries (Italy, Ireland, Germany, France, and Hungary) that aims to better understand how variants in cilia genes cause neurodevelopmental ciliopathies such as Joubert Syndrome. Little is known about how cilia function during nervous system development, and there are no therapies or treatments for the resulting neurodevelopmental abnormalities. This project aims to uncover new knowledge about how Joubert Syndrome variants affect development of cilia and the brain. Ultimately, we hope that this work will lead to better diagnosis, prognosis, and treatment options for people with neurodevelopmental ciliopathies.
The specific aims of NDCil are to:
- Uncover fundamental knowledge of how Joubert Syndrome affects the brain at different levels/scales (eg. cilia, neurons, whole brain)
- Establish how variants found in patients with Joubert Syndrome disrupt cilia function/organisation
- Discover drugs that alleviate Joubert Syndrome symptoms
What are ciliopathies?
Ciliopathies are a group of rare genetic disorders that result from abnormalities in the structure or function of the primary cilium, a hair-like structure that protrudes from the surface of most cells in the body. Primary cilia play critical roles in numerous cellular processes including cell signalling and sensing of mechanical and chemical signals. As a result, defects in primary cilia can lead to a wide range of symptoms and health problems, including vision and hearing loss, kidney disease, skeletal abnormalities, and neurological disorders. Some examples of ciliopathies include Joubert syndrome, Bardet-Biedl syndrome, Alström syndrome, and polycystic kidney disease. Whilst much progress has been made in understanding cilia biology, surprisingly, we know very little about how cilia build and maintain the nervous system, and there are no therapeutic strategies to resolve the neurodevelopmental features of disease.
What is Joubert Syndrome?
Joubert Syndrome is a rare genetic disorder that affects the development of the cerebellum and brainstem, leading to a range of symptoms including breathing abnormalities, problems with coordination and balance, developmental delays, intellectual disability, and vision problems. The disorder is caused by variants in genes involved in cilia function. There are currently no treatments for Joubert Syndrome.
What resources are available for people with Joubert Syndrome?
Joubert Syndrome and Related Disorders Foundation is a global community that can help provide information and support for people with Joubert Syndrome.
Why is this research important?
This research is important because ciliopathies, including Joubert Syndrome, can result in lifelong disabilities, and there are currently no treatments available. By studying how genetic changes affect ciliary function in the brain, this research could lead to new insights into the underlying disease mechanisms and new treatments.
What techniques will be used in this research?
To better understand how changes in genes affect cilia function and brain development, we will employ a range of techniques such as CRISPR gene editing, proteomics and transcriptomics (which analyse proteins and RNA molecules, respectively), 2D and 3D cell imaging, and high throughput drug screening. These techniques will help us to identify and analyse different genetic changes in detail and assess their impact on cilia and neural development.
What research models or organisms will be used?
This research aims to achieve its objectives by using various cell and animal models that replicate the disease state of Joubert Syndrome. These models include pluripotent stem cells from patients, mouse stem cells, zebrafish, and a small worm (C. elegans).
What genes will be investigated in this project?
This project focuses on four genes: TMEM67, CEP290, CC2D2A, and RPGRIP1L. Variants in these four genes are responsible for an estimated 17-35% of Joubert Syndrome cases (Bachmann-Gagescu et al, 2020).
What is a “variant of uncertain significance” (VUS)?
A “variant of uncertain significance” (VUS) is a genetic change that has been identified in an individual’s DNA but its impact on health is unknown or uncertain. In other words, it is a genetic variant whose significance or potential health effects are not yet understood or confirmed. VUS are commonly found in genetic testing, and their uncertain nature can make it difficult for healthcare professionals to interpret and provide accurate diagnoses and treatment recommendations to patients. NDCil will model VUS to generate evidence to determine if the variants cause disease or not.
